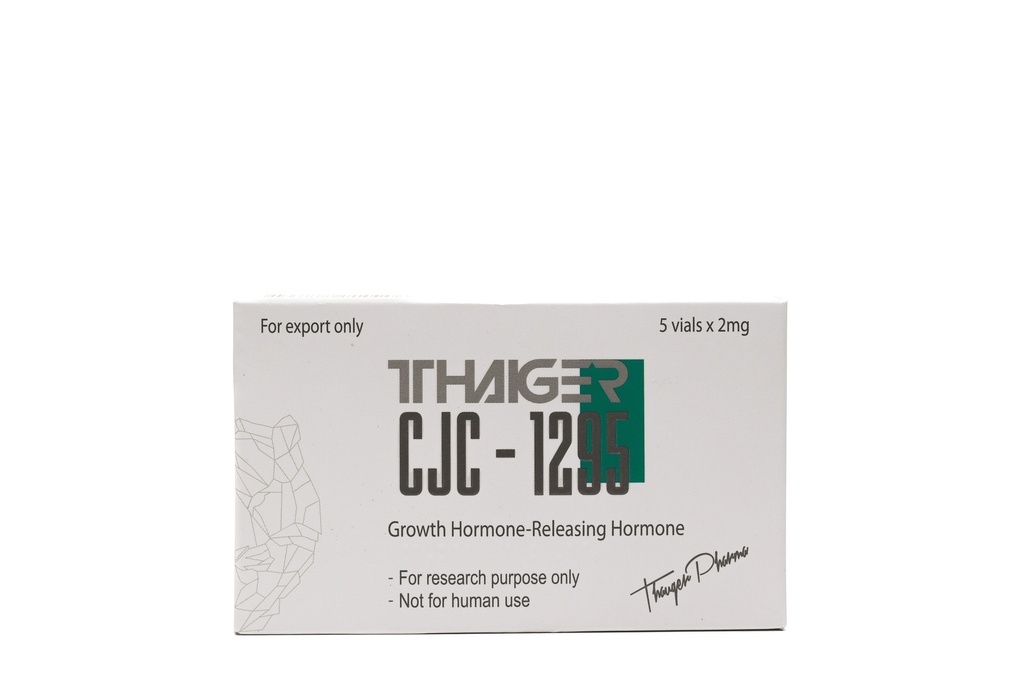
- is a synthetic compound that acts as an analogue of the growth hormone-releasing hormone (GHRH), or somatoliberin, both after a single and multiple administrations. It also increases the level of insulin-like growth factor 1 (IGF-1) in plasma.
- A significant advantage of this compound is that it has been used in comprehensive scientific research on animal models. This enables the estimation of the most effective dosing, potential side effects, and risks associated with its use.
- The peptide was first described in 2006, and its creation is attributed to the Canadian company ConjuChem Biotechnologies.
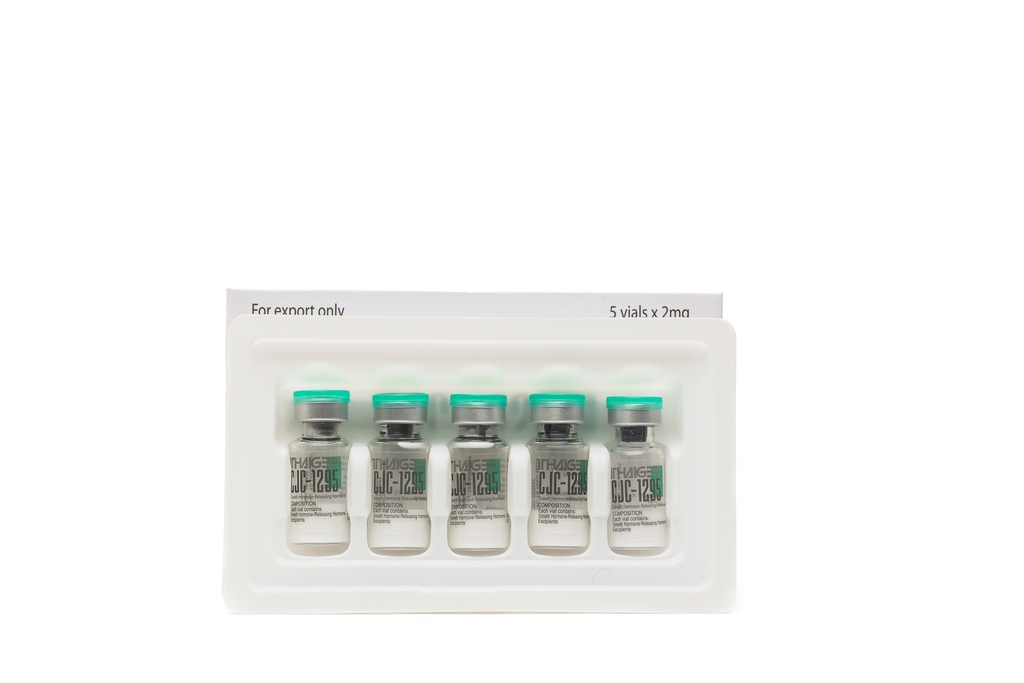
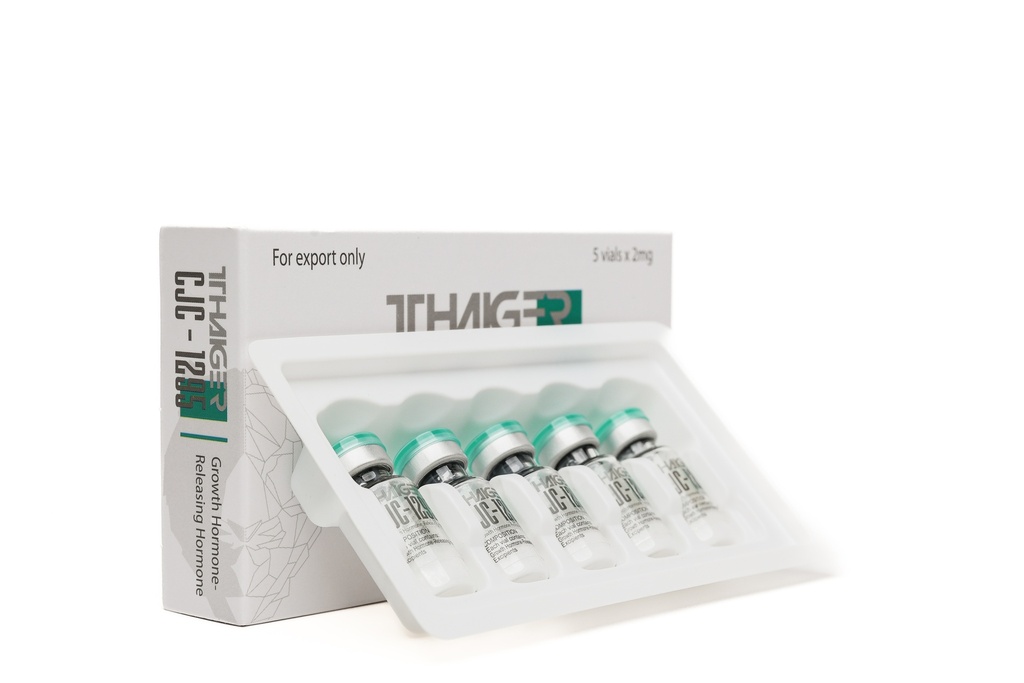
- Composition of the CJC-1295 NO DAC 5mg Preparation
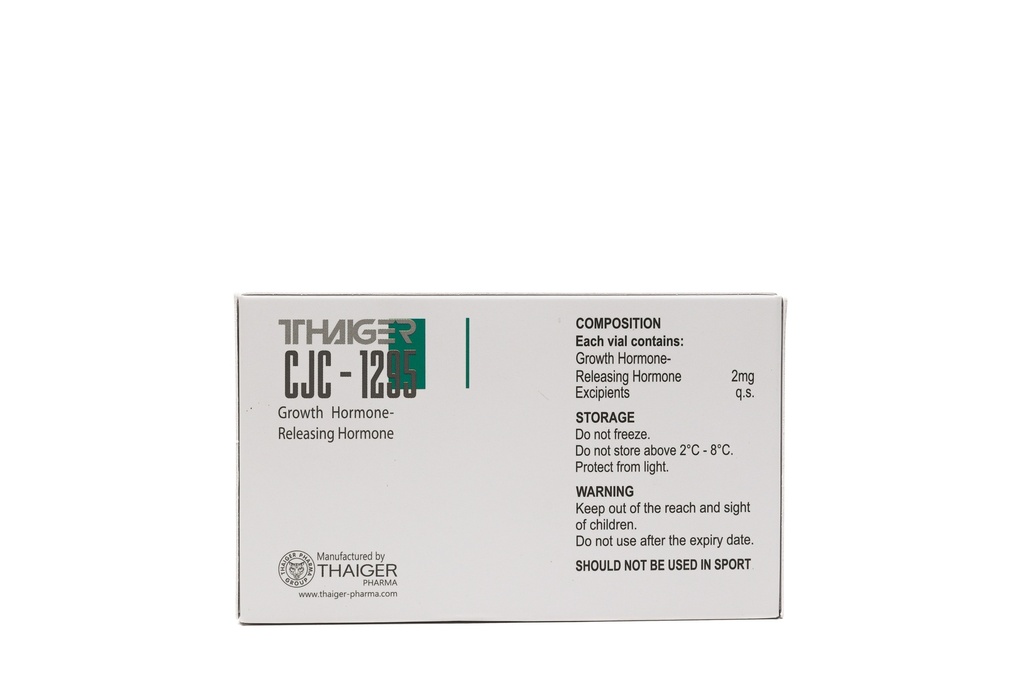
- The CJC-1295 NO DAC2 mg preparation contains 5mg of the active substance. The preparation is distinguished by a high degree of chemical purity and undergoes quality control.
- CJC-1295 5mg is a chemical substance intended exclusively for research purposes.
- In the interest of maintaining the highest quality of the product, laboratory tests regarding concentration and purity are conducted for each batch, the results of which are available in the "Research" section.
- The product is available for sale to private individuals and research institutions, laboratories, universities, or chemical circles aimed at conducting research.
- The product is not a food product or dietary supplement and is not suitable for consumption by humans.
- In case of ill health or adverse reactions caused by contact with the substance, it is advised to seek a doctor's opinion.
- Storage Conditions
- The undiluted vial with lyophilized powder should be stored in a dry, cool, and shaded place. Once dissolved, the substance should be stored at a temperature of 2 to 8°C.
- All the aforementioned properties have been observed in laboratory studies not conducted on humans and are purely informational. All information contained in the descriptions is not approved by GIS, GIF, or EFSA. The substance is not a drug, food product, or dietary supplement and is not intended for human consumption. The product is classified as a chemical reagent/reference material allowed for trade within the European Union and can be used solely for scientific research. Further information about the substance is provided in the chemical safety data sheet, which is available for review. Products are available only to institutions or private individuals engaged in research or laboratory activities.
-
CJC-1295 NO DAC
| العلامة التجارية | Thaiger Pharma |